
Sulfur itself and Li2S are essentially insoluble in the typical electrolyte used in Li/S cells, but these intermediate "polysulfides" often are soluble, which causes an ongoing and severe loss of sulfur at the cathode.
#Sulfur ion charge series#
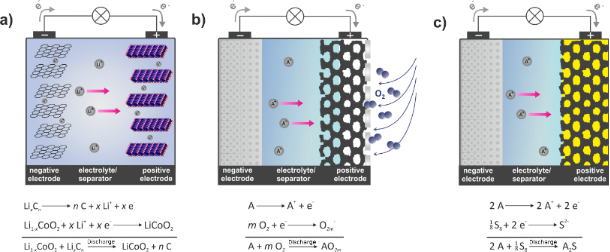
This is a very large source of mechanical stress on the cathode, which causes mechanical deterioration, reduces the electrical contact between the carbon and the sulfur (the path whereby electrons flow to allow the reaction to occur), and prevents the flow of lithium ions to the sulfur surface.Īnother problem is that lithium and sulfur generally don't react immediately to form Li2S, but rather get there through a series of intermediate species, such as Li2S8, Li2S6, etc.

When the sulfur in the cathode absorbs lithium ions from the electrolyte, the Li2S has nearly double the volume of the original sulfur.
The bad news involves a host of materials problems associated with the basic Li/S chemistry and some side reactions. Lithium polysulfides are formed at intermediate charge levels, which affect the cell voltage as indicated above. The flow of two lithium ions from the anode to the cathode is then balanced by the flow of two electrons between the battery contacts, delivering double the current of a Li-ion battery at a voltage between about 1.7 and 2.5 volts, depending on the state of charge of the cell. The overall cell reaction during discharge converts lithium metal in the anode into Li2S at the surface of the cathode. The voltage of a Li/S cell depends on the chemical entities in which electrical energy is stored, which change with the state of charge (Image: Lawrence Berkeley Lab)Ī basic Li/S cell consists of a lithium anode, a carbon-sulfur cathode, and an electrolyte that permits lithium ions to pass.


 0 kommentar(er)
0 kommentar(er)
